
Chemistry, 18.03.2021 02:10 krystlemiller11211
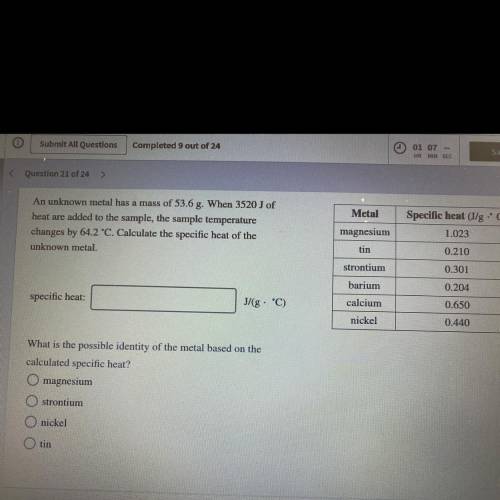
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp. changes by 64.2°C. Calculate specific heat of the unknown metal. what is the metal?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp....
Questions


Mathematics, 18.03.2021 03:00


Biology, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


Social Studies, 18.03.2021 03:00


History, 18.03.2021 03:00



English, 18.03.2021 03:00

Geography, 18.03.2021 03:00



Social Studies, 18.03.2021 03:00

English, 18.03.2021 03:00




