
Chemistry, 18.03.2021 02:00 boogerbuttday
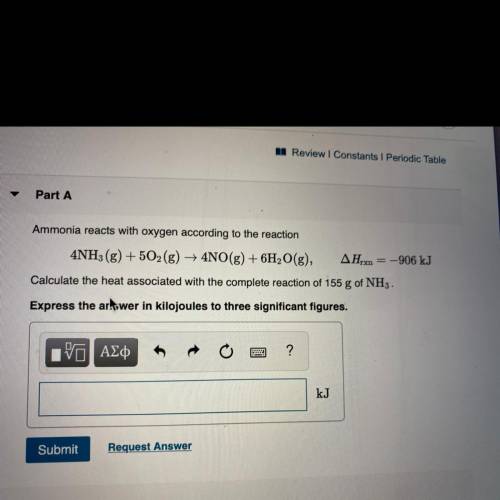
Ammonia reacts with oxygen according to the reaction
4NH3(g) + 5O2(g) + 4NO(g) + 6H2O(g), AHrx = -906 kJ
Calculate the heat associated with the complete reaction of 155 g of NH3 .
Express the artswer in kilojoules to three significant figures.
Se
ΑΣΦ
?
kJ
her
9.pdf
Submit
Request Answer
Part B

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
Ammonia reacts with oxygen according to the reaction
4NH3(g) + 5O2(g) + 4NO(g) + 6H2O(g), AHrx = -9...
Questions








Mathematics, 10.11.2020 17:00

Advanced Placement (AP), 10.11.2020 17:00



Mathematics, 10.11.2020 17:00



English, 10.11.2020 17:00







