
Chemistry, 18.03.2021 01:40 baileyanne9389
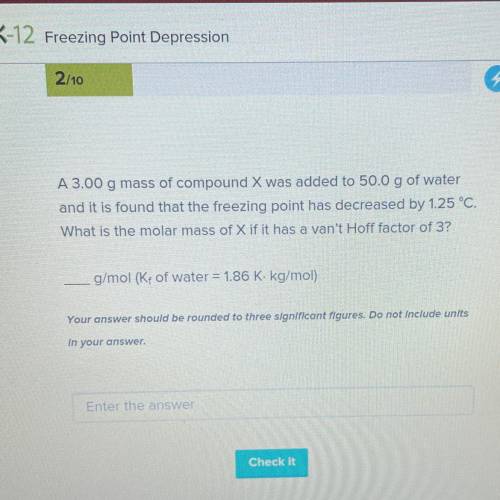
A 3.00 g mass of compound X was added to 50.0 g of water
and it is found that the freezing point has decreased by 1.25 °C.
What is the molar mass of X if it has a van't Hoff factor of 3?
g/mol (K, of water = 1.86 K. kg/mol)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
A 3.00 g mass of compound X was added to 50.0 g of water
and it is found that the freezing point ha...
Questions

English, 16.12.2020 02:50



Mathematics, 16.12.2020 02:50

History, 16.12.2020 02:50

Physics, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50

Chemistry, 16.12.2020 02:50








Mathematics, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50




