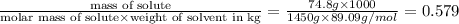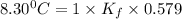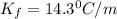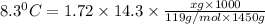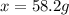Chemistry, 18.03.2021 01:20 Drevei6969
When 74.8g of alanine C3H7NO2 are dissolved in 1450.g of a certain mystery liquid X, the freezing point of the solution is 8.30°C less than the freezing point of pure X. Calculate the mass of potassium bromide that must be dissolved in the same mass of X to produce the same depression in freezing point. The van't Hoff factor =i1.72 for potassium bromide in X.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1


Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
When 74.8g of alanine C3H7NO2 are dissolved in 1450.g of a certain mystery liquid X, the freezing po...
Questions

Chemistry, 07.12.2021 03:00


SAT, 07.12.2021 03:00




Chemistry, 07.12.2021 03:00


English, 07.12.2021 03:00


Business, 07.12.2021 03:00



SAT, 07.12.2021 03:00






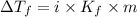
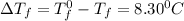 = Depression in freezing point
= Depression in freezing point
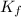 = freezing point constant =
= freezing point constant =