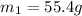Chemistry, 18.03.2021 01:10 katherineweightman
A sample of aluminum, which has a specific heat capacity of 0.897·J·g−1°C^−1 , is put into a calorimeter (see sketch at right) that contains 300.0g of water. The aluminum sample starts off at 94.5°C and the temperature of the water starts off at 21.0°C .When the temperature of the water stops changing it's 23.8°C .The pressure remains constant at 1atm .Calculate the mass of the aluminum sample.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2


You know the right answer?
A sample of aluminum, which has a specific heat capacity of 0.897·J·g−1°C^−1 , is put into a calorim...
Questions

Mathematics, 10.03.2021 20:30


Mathematics, 10.03.2021 20:30

Mathematics, 10.03.2021 20:30

Mathematics, 10.03.2021 20:30


Mathematics, 10.03.2021 20:30

History, 10.03.2021 20:30

History, 10.03.2021 20:30



Mathematics, 10.03.2021 20:30

English, 10.03.2021 20:30

SAT, 10.03.2021 20:30

Chemistry, 10.03.2021 20:30

Mathematics, 10.03.2021 20:30

Mathematics, 10.03.2021 20:30


History, 10.03.2021 20:30

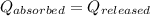
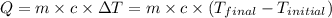
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/1195/8097/b0e58.png)
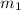 = mass of aluminium = ?
= mass of aluminium = ?
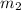 = mass of water = 300.0 g
= mass of water = 300.0 g
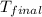 = final temperature =
= final temperature = 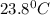
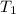 = temperature of aluminium =
= temperature of aluminium = 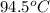
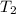 = temperature of water =
= temperature of water = 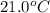
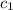 = specific heat of aluminium =
= specific heat of aluminium = 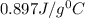
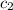 = specific heat of water =
= specific heat of water = 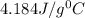
![-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1195/8097/92a72.png)
![-[m_1\times 0.897\times (23.8-94.5)^0C]=[300.0g\times 4.184\times (23.8-21.0)]](/tpl/images/1195/8097/6f259.png)