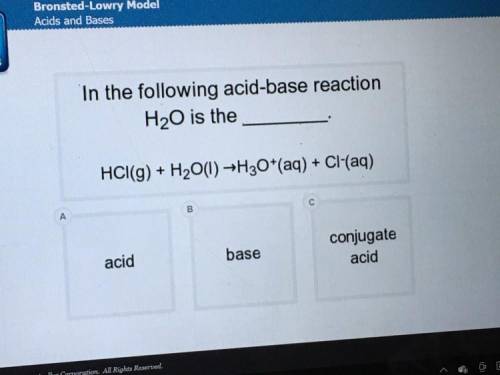
In the following acid-base reaction, h20 is the_ hci(g)+h2o(I) = h3o+(aq)+ci-(aq)
...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
Questions


Mathematics, 27.12.2019 17:31

English, 27.12.2019 17:31

Mathematics, 27.12.2019 17:31

History, 27.12.2019 17:31



Mathematics, 27.12.2019 17:31

History, 27.12.2019 17:31

Mathematics, 27.12.2019 17:31


Physics, 27.12.2019 17:31


Arts, 27.12.2019 17:31

Business, 27.12.2019 17:31

Spanish, 27.12.2019 17:31