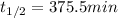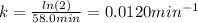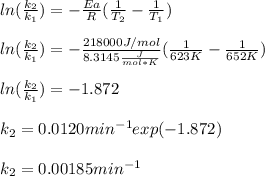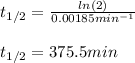Chemistry, 18.03.2021 01:00 21ghostrider21
The decomposition of ethylene oxide (CH₂)₂O(g) → CH₄(g) + CO(g) is a first order reaction with a half-life of 58.0 min at 652 K. The activation energy of the reaction is 218 kJ/mol. Calculate the half-life at 623 K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3


Chemistry, 23.06.2019 10:30
What are two radioactive isotopes that are used for dating rocks that are older than 10 million years
Answers: 2

Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2
You know the right answer?
The decomposition of ethylene oxide (CH₂)₂O(g) → CH₄(g) + CO(g) is a first order reaction with a hal...
Questions

Health, 08.12.2020 22:40

Mathematics, 08.12.2020 22:40



Mathematics, 08.12.2020 22:40

Mathematics, 08.12.2020 22:40


Business, 08.12.2020 22:40


Biology, 08.12.2020 22:40



History, 08.12.2020 22:40

Mathematics, 08.12.2020 22:40

History, 08.12.2020 22:40

English, 08.12.2020 22:40

Mathematics, 08.12.2020 22:40


Business, 08.12.2020 22:40

English, 08.12.2020 22:40