
Chemistry, 17.03.2021 23:50 TrueMonster8911
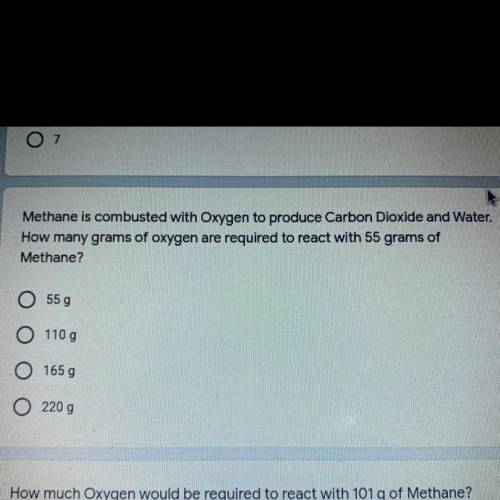
Methane is combusted with Oxygen to produce Carbon Dioxide and Water. How many grams of oxygen are required to react with 55 grams of
Methane?
A.55 g
B.110 g
C.165 g
D.220 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
Methane is combusted with Oxygen to produce Carbon Dioxide and Water. How many grams of oxygen are r...
Questions

Mathematics, 03.05.2020 13:15

History, 03.05.2020 13:15


Mathematics, 03.05.2020 13:15


Computers and Technology, 03.05.2020 13:15



Mathematics, 03.05.2020 13:15



Mathematics, 03.05.2020 13:15

Mathematics, 03.05.2020 13:15


Mathematics, 03.05.2020 13:15

Mathematics, 03.05.2020 13:15


English, 03.05.2020 13:15

Mathematics, 03.05.2020 13:15





