
Chemistry, 17.03.2021 23:40 nataliecooper542
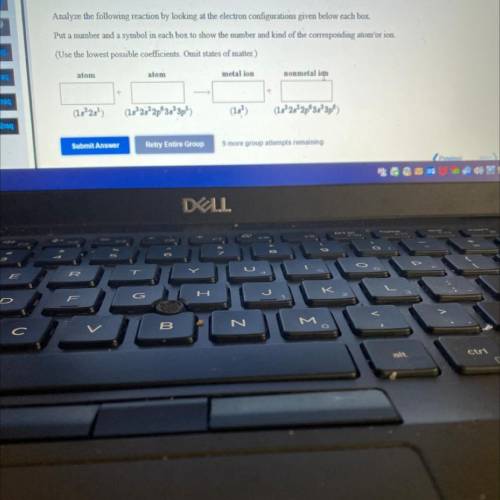
Analyze the following reaction by looking at the electron configurations given below each box.
pts
Put a number and a symbol in each box to show the number and kind of the corresponding atomʻor ion.
pts 2reg
(Use the lowest possible coefficients. Omit states of matter.)
pts 2req
atom
atom
metal ion
nonmetal ion
pts 2reg
(182)
(1s 2s 2p 3s 3p5)
(15)
(15°25² 2p 3s 3p
1 pts 2rea

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Analyze the following reaction by looking at the electron configurations given below each box.
pts<...
Questions




Social Studies, 02.10.2019 01:30

Chemistry, 02.10.2019 01:30


Mathematics, 02.10.2019 01:30

Mathematics, 02.10.2019 01:30

Mathematics, 02.10.2019 01:30






English, 02.10.2019 01:30

Mathematics, 02.10.2019 01:30


Mathematics, 02.10.2019 01:30

History, 02.10.2019 01:30



