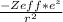Chemistry, 13.03.2021 05:50 HeyItsCookie9605
Describe the critical property ofelectrons in 2s versus 2p orbitals that causes the radial distribution functions of these orbitals to have different shapes about the nucleus such that 2s electrons effectively penetrate closer to the nucleus than do electrons in 2p orbitals. Then, write an expression for the potential energy function describing this effect.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
You know the right answer?
Describe the critical property ofelectrons in 2s versus 2p orbitals that causes the radial distribut...
Questions

Mathematics, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00




Mathematics, 16.12.2020 01:00

History, 16.12.2020 01:00


English, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00


Chemistry, 16.12.2020 01:00


Mathematics, 16.12.2020 01:00

History, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Law, 16.12.2020 01:00

Biology, 16.12.2020 01:00