a. neutrons and electrons

Chemistry, 01.01.2020 00:31 ayaanwaseem
1. which two particles are found in the nucleus of an atom?
a. neutrons and electrons
b. protons and electrons
c. protons and neutrons
d. neutrons and atoms
2. the table below gives the atomic mass and relative abundance values for the three isotopes of element m.
what is the average atomic mass (in amu) of element m?
a.2.86
b.5.36
c.24.30
d.24.98
relative abundance (%) atomic mass (amu)
78.99 23.9850
10.00 24.9858
11.01 25.9826
3. a particle that orbits the nucleus in an atom is called a .
4. the table below gives the numbers of protons, electrons, and neutrons in four atoms.
atom number of protons number of neutrons number of electrons
1 9 8 8
2 9 9 8
3 9 9 10
4 9 10 9
based on the table, which atom has a charge of –1?
a.1
b.2
c.3
d.4
5. boron occurs naturally as two isotopes. what is the difference between these isotopes?
a. they have different numbers of electrons and different charges.
b. they have different numbers of neutrons and different charges.
c. they have different numbers of protons and different mass numbers.
d. they have different numbers of neutrons and different mass numbers.
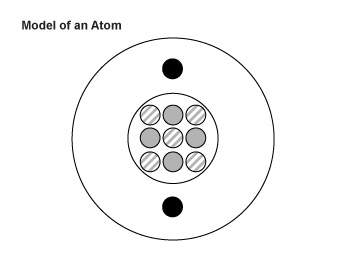
6. the diagram below is an artist’s impression of a single atom of element be. the neutrons are shown with stripes, the protons are gray, and the electrons are black. these particles are not drawn to scale.
which element below could be an isotope of this atom?
a. sodium-10
b. beryllium-10
c. boron-9
d. carbon-9
7. the mass number of fe2+ is 56. how many neutrons are there in a single fe2+ atom?
a. 28
b. 30
c. 56
d. 58
8. a scientist uses an accelerator and high energy electrons to study the particles inside the protons of a helium atom. what particles is the scientist studying?
a. the he neutrons
b. the he nucleus
c. the he electrons
d. the he quarks
9. which element has 16 protons in its nucleus?
a. neon
b. oxygen
c. sodium
d. sulfur
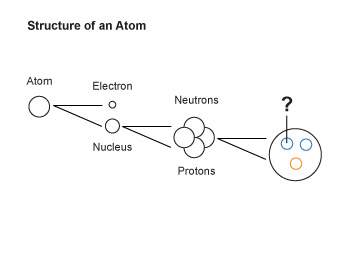
10. the diagram below shows some subatomic particles.
what is the particle that is labeled with a question mark in the diagram?
a. beta particle
b. quark
c. isotope
d. alpha particle


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3
You know the right answer?
1. which two particles are found in the nucleus of an atom?
a. neutrons and electrons
a. neutrons and electrons
Questions





English, 04.09.2020 01:01




Social Studies, 04.09.2020 01:01

Computers and Technology, 04.09.2020 01:01

Computers and Technology, 04.09.2020 01:01


Chemistry, 04.09.2020 01:01






Biology, 04.09.2020 01:01




