
Chemistry, 12.03.2021 22:00 Michael321
How would i set this up to find specific heat capacity (c) of copper? (Cu)
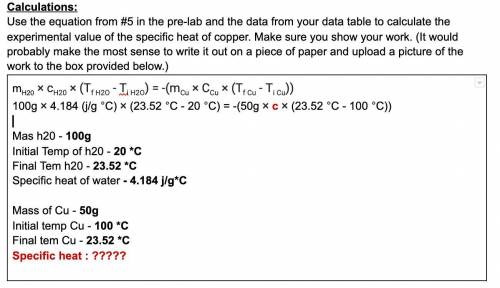
mH20 × cH20 × (ΔT H20) = -(m Cu × C Cu × (ΔT Cu))
100g × 4.184 (j/g °C) × (23.52 °C - 20 °C) = -(50g × c cu × (23.52 °C - 100 °C))

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3


Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
How would i set this up to find specific heat capacity (c) of copper? (Cu)
mH20 × cH20 × (ΔT H20) =...
Questions


Biology, 15.12.2020 19:40

English, 15.12.2020 19:40



Mathematics, 15.12.2020 19:40

English, 15.12.2020 19:40


French, 15.12.2020 19:40


Mathematics, 15.12.2020 19:40


English, 15.12.2020 19:40

History, 15.12.2020 19:40

Mathematics, 15.12.2020 19:40

Spanish, 15.12.2020 19:40



Mathematics, 15.12.2020 19:40

English, 15.12.2020 19:40



