Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
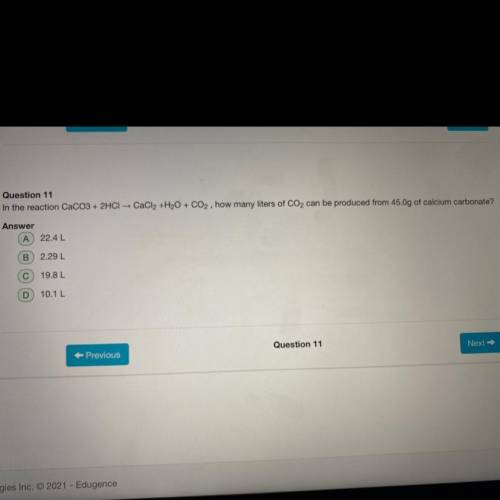
In the reaction CaCO3+2HCl CaCl 2 +H 2 O+CO 2 how many liters of CO 2 can be produced from 45.0g of...
Questions




English, 02.12.2019 15:31

Mathematics, 02.12.2019 15:31

Mathematics, 02.12.2019 15:31


Mathematics, 02.12.2019 15:31

Mathematics, 02.12.2019 15:31


Biology, 02.12.2019 15:31

Mathematics, 02.12.2019 15:31



Mathematics, 02.12.2019 15:31


Mathematics, 02.12.2019 15:31


History, 02.12.2019 15:31