
Chemistry, 12.03.2021 18:20 savannahvargas512
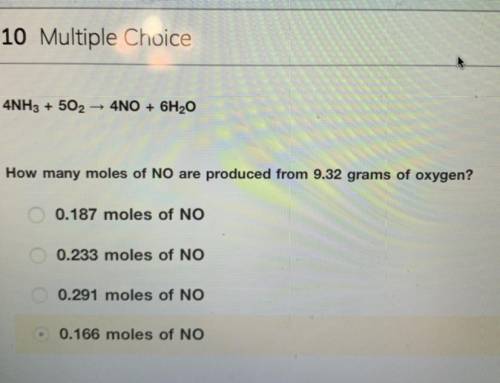
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
the answer is not 0.166 moles i tried and did not get credit for.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3


Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
4NH3 + 5O2 > 4NO + 6H2O
How many moles of NO are produced from 9.32 grams of oxygen?
Questions

English, 27.03.2021 21:10

English, 27.03.2021 21:20



History, 27.03.2021 21:20

Mathematics, 27.03.2021 21:20

Business, 27.03.2021 21:20





Mathematics, 27.03.2021 21:20



Biology, 27.03.2021 21:20



Mathematics, 27.03.2021 21:20

Chemistry, 27.03.2021 21:20



