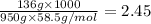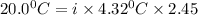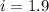Chemistry, 12.03.2021 15:30 Cnolteb5663
When 136g of glycine are dissolved in of a certain mystery liquid , the freezing point of the solution is lower than the freezing point of pure . On the other hand, when of sodium chloride are dissolved in the same mass of , the freezing point of the solution is lower than the freezing point of pure . Calculate the van't Hoff factor for sodium chloride in . Be sure your answer has a unit symbol, if necessary, and round your answer to significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1


Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
When 136g of glycine are dissolved in of a certain mystery liquid , the freezing point of the soluti...
Questions

Mathematics, 02.06.2020 10:57


Mathematics, 02.06.2020 10:57

Mathematics, 02.06.2020 10:57

Mathematics, 02.06.2020 10:57


History, 02.06.2020 10:57




Physics, 02.06.2020 10:57

Mathematics, 02.06.2020 10:57

Mathematics, 02.06.2020 10:57




Mathematics, 02.06.2020 10:57



Mathematics, 02.06.2020 10:57

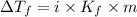
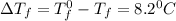 = Depression in freezing point
= Depression in freezing point
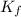 = freezing point constant
= freezing point constant 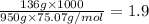
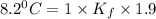
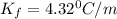
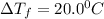 = Depression in freezing point
= Depression in freezing point