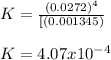Chemistry, 12.03.2021 15:20 pickelswolf3962
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni(CO)4(g) → Ni(s)+ 4CO(g)
At a certain temperature, a chemist finds that a 2.6 L reaction vessel containing a mixture of nickel carbonyl, nickel, and carbon monoxide at equilibrium has the following composition:
compound amount
Ni(CO)4 0.597g
Ni 12.7g
CO 1.98g
Required:
Calculate the value of the equilibrium constant for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni(CO)4(g) → Ni(s)+ 4CO(g...
Questions


Mathematics, 18.12.2020 02:50


Social Studies, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50



Chemistry, 18.12.2020 02:50

English, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50



Mathematics, 18.12.2020 02:50


Mathematics, 18.12.2020 02:50


History, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Computers and Technology, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

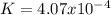
![K=\frac{[CO]^4}{[Ni(CO)_4]}](/tpl/images/1190/9822/9636a.png)
![[CO]_{EQ}=\frac{1.98g}{28.01g/mol} *\frac{1}{2.6L}=0.0272M](/tpl/images/1190/9822/0dde7.png)
![[Ni(CO)_4]_{EQ}=\frac{0.597g}{170.73g/mol} *\frac{1}{2.6L}=0.001345M](/tpl/images/1190/9822/2339a.png)