
Chemistry, 12.03.2021 08:30 Conner5459
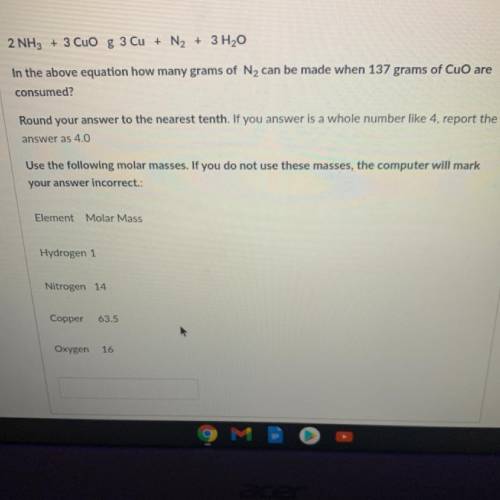
2 NH3 + 3 CuO g 3 Cu + N2 + 3 H2O
In the above equation how many grams of N2 can be made when 137 grams of CuO are
consumed?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
2 NH3 + 3 CuO g 3 Cu + N2 + 3 H2O
In the above equation how many grams of N2 can be made when 137 g...
Questions


Mathematics, 05.11.2020 22:30



History, 05.11.2020 22:30


Mathematics, 05.11.2020 22:30

Health, 05.11.2020 22:30

Mathematics, 05.11.2020 22:30

Arts, 05.11.2020 22:30

Mathematics, 05.11.2020 22:30

Mathematics, 05.11.2020 22:30

Arts, 05.11.2020 22:30






Mathematics, 05.11.2020 22:30



