
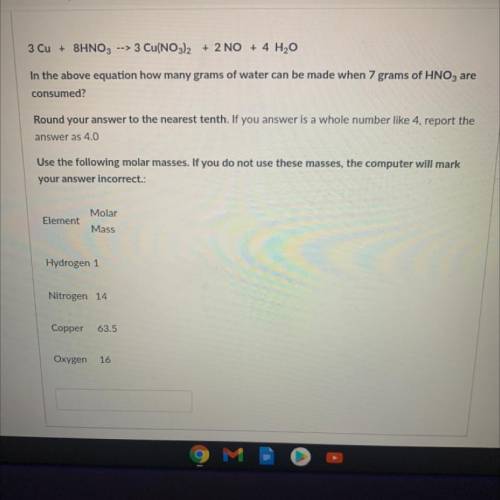
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many grams of water can be made when 7 grams of HNO3 are
consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the
answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark
your answer incorrect.:
Element
Molar
Mass
Hydrogen 1
Nitrogen 14
Copper
63.5
Oxygen
16

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many grams of water can be...
Questions



Mathematics, 19.09.2019 01:30





Biology, 19.09.2019 01:30

Mathematics, 19.09.2019 01:30

Health, 19.09.2019 01:30

Mathematics, 19.09.2019 01:30



Mathematics, 19.09.2019 01:30

Mathematics, 19.09.2019 01:30

Business, 19.09.2019 01:30

Computers and Technology, 19.09.2019 01:30



English, 19.09.2019 01:30



