
Chemistry, 12.03.2021 07:10 nathangirnet
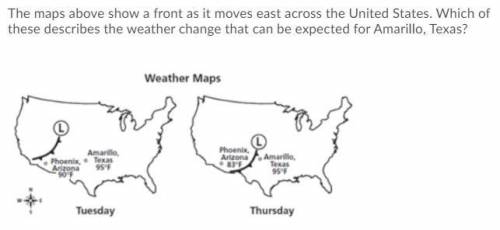
The maps above show a front as it moves east across the United States. Which of these describes the weather change that can be expected for Amarillo, Texas?
A. The cold front will bring cooler temperatures and snow because the cold front is producing large amounts of energy and snow moving southeast.
B. The warm front will bring warmer temperatures with hot air for several days because it pushes the cold air out of the way.
C. The warm front will bring warmer temperatures with light rain for a few days because the warm air rises and cools producing rain.
D. The cold front will bring cooler temperatures with thunderstorms and rain, which causes the moisture in the warm air to condense and release large amounts of energy.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

You know the right answer?
The maps above show a front as it moves east across the United States. Which of these describes the...
Questions

Mathematics, 29.10.2019 02:31




English, 29.10.2019 02:31




Mathematics, 29.10.2019 02:31

Mathematics, 29.10.2019 02:31

Mathematics, 29.10.2019 02:31



History, 29.10.2019 02:31



Mathematics, 29.10.2019 02:31


English, 29.10.2019 02:31



