C2H4(g) + 3O2(g) —> 2CO2(g) + 2H2O(g)
change in H1 = ?
The combustion of C2H4 is rep...

Chemistry, 12.03.2021 03:00 rustjallison9928
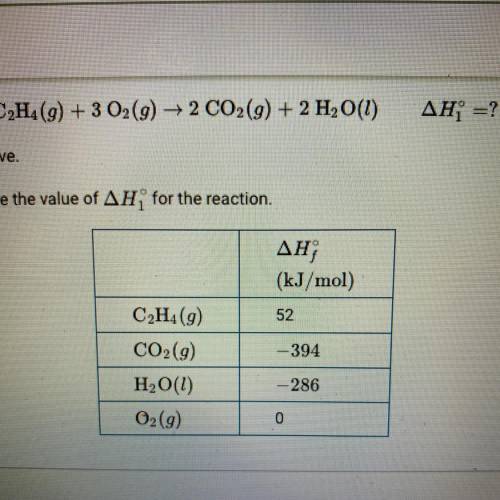
C2H4(g) + 3O2(g) —> 2CO2(g) + 2H2O(g)
change in H1 = ?
The combustion of C2H4 is represented by the equation above.
Use the enthalpies of formation in the table the calculate the value of change in H1 for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
Questions

History, 14.12.2020 18:30

English, 14.12.2020 18:30

History, 14.12.2020 18:30



History, 14.12.2020 18:30

Mathematics, 14.12.2020 18:30


Mathematics, 14.12.2020 18:30

Mathematics, 14.12.2020 18:30




Mathematics, 14.12.2020 18:30


Social Studies, 14.12.2020 18:30


History, 14.12.2020 18:30




