
Chemistry, 12.03.2021 01:00 91miketaylor
The Bohr model of an Atom protons are represented in blue the neutrons are red and the electrons are green which color circle would you use to determine the identity of the atom
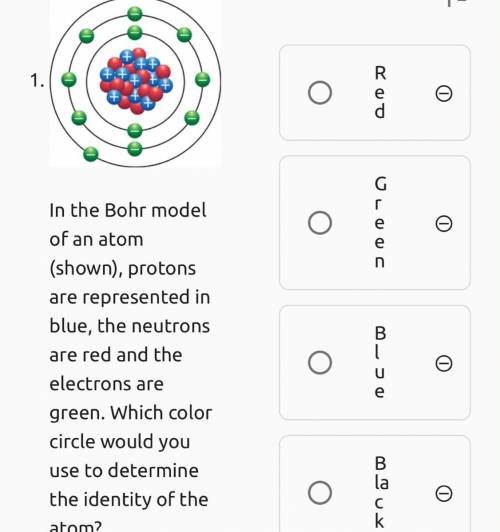

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
The Bohr model of an Atom protons are represented in blue the neutrons are red and the electrons are...
Questions



Mathematics, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01

History, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01

Spanish, 02.07.2020 01:01



English, 02.07.2020 01:01


Biology, 02.07.2020 01:01

History, 02.07.2020 01:01








