
Can someone help me please
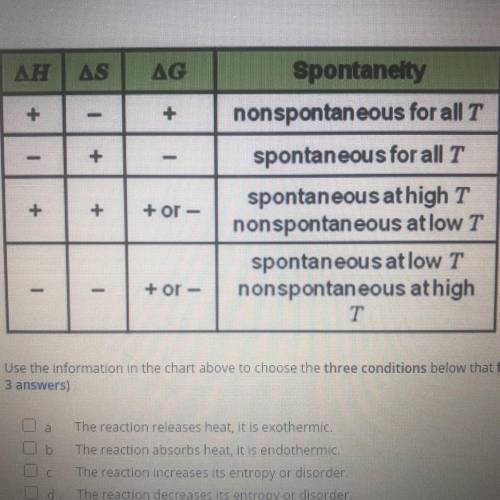
using the information in the chart above to choose the three conditions below that favor spontaneous reaction at all temperaturs ( Choose 3 answers)
A. The reaction release heat it is exothermic
B. The reaction absorbs heat it is endothermic
C. The reaction increases its entropy or disorder
D. The reaction decreases its entropy or disorder
E. The value of G is negative
F. The value of G is positive

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Can someone help me please
using the information in the chart above to choose the three conditions...
Questions

Mathematics, 20.11.2020 17:50


Mathematics, 20.11.2020 17:50

Arts, 20.11.2020 17:50

English, 20.11.2020 17:50


History, 20.11.2020 17:50


English, 20.11.2020 17:50

English, 20.11.2020 17:50

Chemistry, 20.11.2020 17:50


Geography, 20.11.2020 17:50



Mathematics, 20.11.2020 17:50

Mathematics, 20.11.2020 17:50


Mathematics, 20.11.2020 17:50




