
Can someone help me please
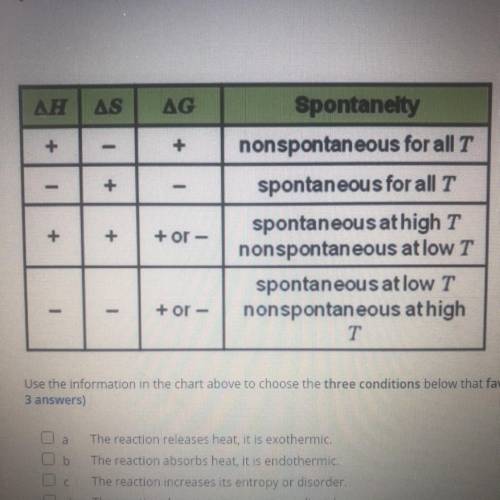
using the information in the chart above to choose the three conditions below that favor spontaneous reaction at all temperaturs ( Choose 3 answers)
A. The reaction release heat it is exothermic
B. The reaction absorbs heat it is endothermic
C. The reaction increases its entropy or disorder
D. The reaction decreases its entropy or disorder
E. The value of G is negative
F. The value of G is positive

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Can someone help me please
using the information in the chart above to choose the three conditions...
Questions






Mathematics, 16.04.2020 00:04


Mathematics, 16.04.2020 00:04

History, 16.04.2020 00:04

Mathematics, 16.04.2020 00:04

Mathematics, 16.04.2020 00:04

English, 16.04.2020 00:04

Mathematics, 16.04.2020 00:04





Biology, 16.04.2020 00:04




