35 POINTS
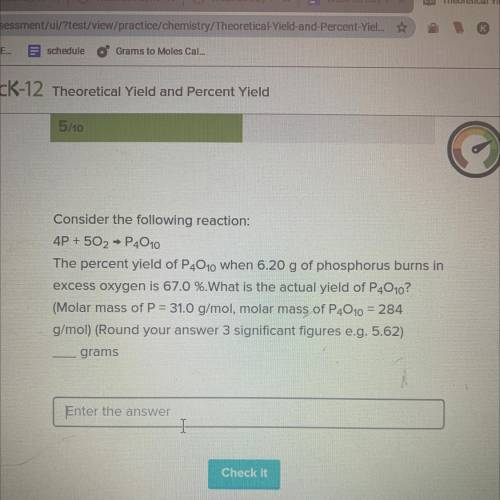
Consider the following reaction:
4P + 502 → P4010
The percent yield of P4010...

Chemistry, 11.03.2021 19:30 dontcareanyonemo
35 POINTS
Consider the following reaction:
4P + 502 → P4010
The percent yield of P4010 when 6.20 g of phosphorus burns in
excess oxygen is 67.0 %.What is the actual yield of P4010?
(Molar mass of P = 31.0 g/mol, molar mass of P4010 = 284
g/mol) (Round your answer 3 significant figures e. g. 5.62)
grams

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3
You know the right answer?
Questions




Mathematics, 20.12.2020 06:40


English, 20.12.2020 06:40



Engineering, 20.12.2020 06:50


Mathematics, 20.12.2020 06:50




Mathematics, 20.12.2020 06:50

Mathematics, 20.12.2020 06:50

English, 20.12.2020 06:50


Mathematics, 20.12.2020 06:50

Mathematics, 20.12.2020 06:50



