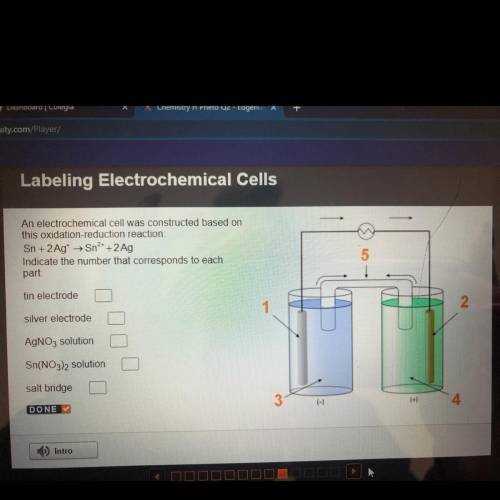
An electrochemical cell was constructed based on
this oxidation-reduction reaction:
Sn + 2 Ag...

Chemistry, 11.03.2021 18:00 emacwhaleng
An electrochemical cell was constructed based on
this oxidation-reduction reaction:
Sn + 2 Ag+ + Sn2+ + 2 Ag
Indicate the number that corresponds to each
part:
tin electrode
silver electrode
AgNO3 solution
Sn(NO3)2 solution
salt bridge

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Questions



Mathematics, 08.01.2021 02:40








English, 08.01.2021 02:40

Mathematics, 08.01.2021 02:40

History, 08.01.2021 02:40



Mathematics, 08.01.2021 02:40


Physics, 08.01.2021 02:40

Mathematics, 08.01.2021 02:40



