Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
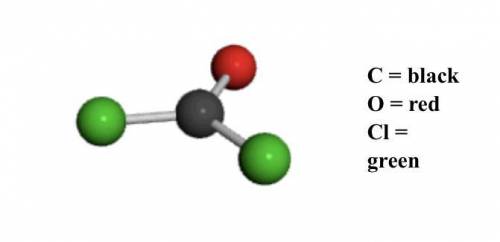
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model b...
Questions

Mathematics, 06.02.2022 20:50




German, 06.02.2022 20:50


Health, 06.02.2022 20:50

Chemistry, 06.02.2022 20:50

English, 06.02.2022 20:50



Geography, 06.02.2022 20:50


Mathematics, 06.02.2022 20:50

Chemistry, 06.02.2022 20:50

Chemistry, 06.02.2022 20:50


English, 06.02.2022 21:00


Biology, 06.02.2022 21:00