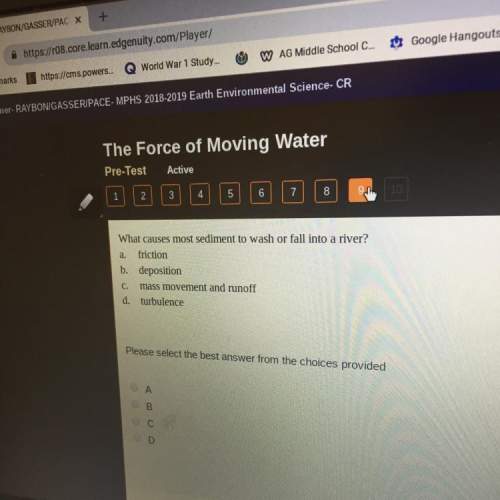
Chemistry, 11.03.2021 06:40 corbin3582
A buffer consists of 0.11 M NaH2PO4 and 0.38 M Na2HPO4. Phosphoric acid is a triprotic acid
(Ka1 = 7.2 x 10-3, K42 = 6.3 x 10-8, K13 = 4.2 x 10-13).
(a) Which K, value is most important to this buffer?
O
Kq1 is most important.
O
K 2 is most important.
O
K 3 is most important.
(b) What is the buffer pH?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
A buffer consists of 0.11 M NaH2PO4 and 0.38 M Na2HPO4. Phosphoric acid is a triprotic acid
(Ka1 =...
Questions



Mathematics, 12.08.2020 22:01




English, 12.08.2020 22:01


Mathematics, 12.08.2020 22:01




English, 12.08.2020 22:01

Mathematics, 12.08.2020 22:01

Biology, 12.08.2020 22:01




Mathematics, 12.08.2020 22:01