
Chemistry, 11.03.2021 05:50 ilovemusicandreading
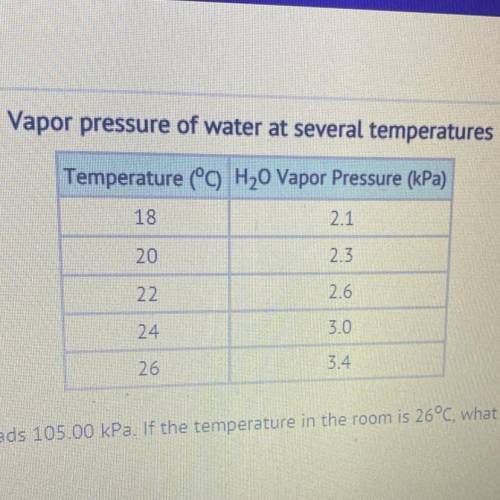
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is the partial pressure of the dry
air??
A
30.88 kPa
B)
101.60 kPa
108.40 kPa
D)
357.00 kPa

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is th...
Questions

Mathematics, 31.07.2019 04:30









Mathematics, 31.07.2019 04:30


Mathematics, 31.07.2019 04:30




English, 31.07.2019 04:30

English, 31.07.2019 04:30



Geography, 31.07.2019 04:30



