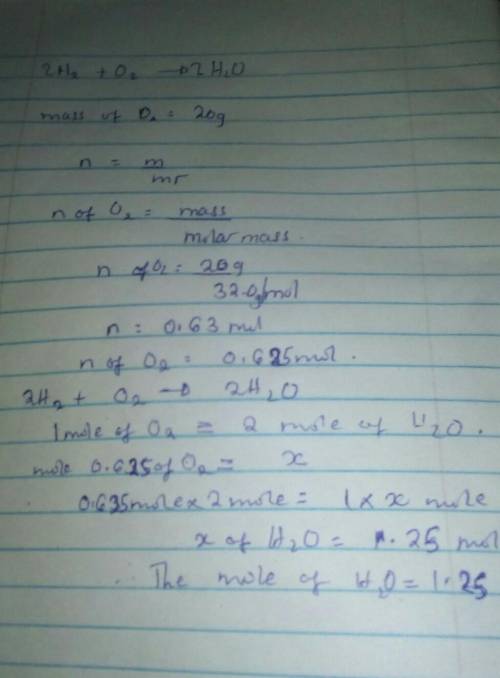Chemistry, 10.03.2021 23:00 trinidymwilga
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start with 20 grams of Oz, how many moles of H2O can be made? 1.25 mol 320 mol 1280 mol 0.31 mol Question 9 (1 point)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start...
Questions



History, 13.11.2020 21:20

Biology, 13.11.2020 21:20

Health, 13.11.2020 21:20

Health, 13.11.2020 21:20

History, 13.11.2020 21:20


Chemistry, 13.11.2020 21:20




Chemistry, 13.11.2020 21:20

Mathematics, 13.11.2020 21:20

History, 13.11.2020 21:20

Advanced Placement (AP), 13.11.2020 21:20



History, 13.11.2020 21:20