Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3

Chemistry, 23.06.2019 09:20
Four statements about the development of the atomic model are shown below. a: electrons have wavelike properties. b: atoms have small, negatively charged particles. c. the center of an atom is a small, dense nucleus. d: atoms are hard, indivisible spheres. which order of statements represents the historical development of the atomic model? c-d-a-b c-d-b-a d— в-а — с d-b-c-a
Answers: 1
You know the right answer?
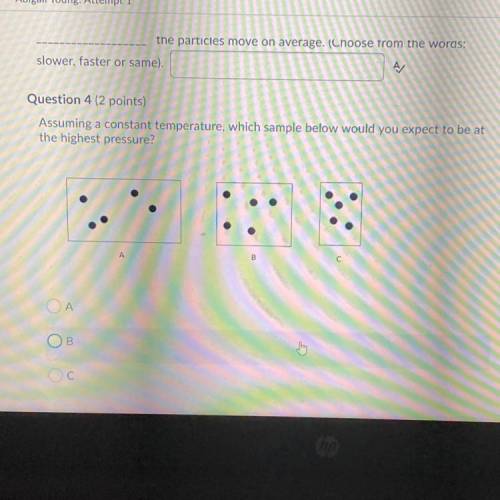
Assuming a constant temperature, which sample below would you expect to be at
the highest pressure?...
Questions

Mathematics, 06.12.2019 08:31

History, 06.12.2019 08:31

Health, 06.12.2019 08:31

Business, 06.12.2019 08:31

History, 06.12.2019 08:31

Mathematics, 06.12.2019 08:31

English, 06.12.2019 08:31


Mathematics, 06.12.2019 08:31

Mathematics, 06.12.2019 08:31


Biology, 06.12.2019 08:31

Chemistry, 06.12.2019 08:31

Mathematics, 06.12.2019 08:31

History, 06.12.2019 08:31

Biology, 06.12.2019 08:31

Mathematics, 06.12.2019 08:31