Write your answer below.

Chemistry, 10.03.2021 20:20 LillianMRucker
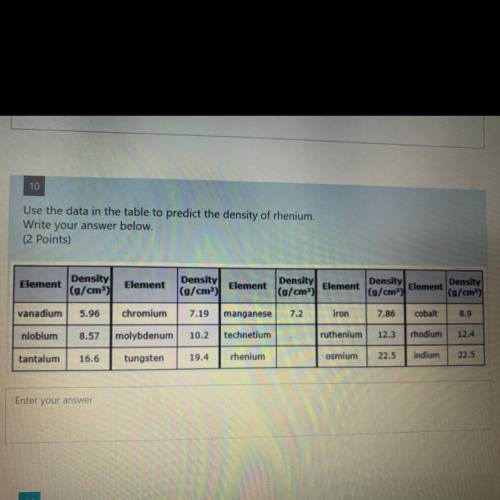
10
Use the data in the table to predict the density of rhenium.
Write your answer below.
(2 points)
Element
Density
(g/cm)
Element
Density
(g/cm)
Element
Density
Element
Density
(g/cm)
Element
(g/cm)
Density
(g/cm”)
vanadium
5.96
chromium
7.19
manganese
7.2
iron
7.86
cobalt
8.9
niobium
8.57
molybdenum
10.2
technetium
ruthenium
12.3
rhodium
12.4
tantalum
16.6
tungsten
19.4
rhenium
osmium
22.5
indium
22.5
Enter your answer

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
10
Use the data in the table to predict the density of rhenium.
Write your answer below.
Write your answer below.
Questions








History, 03.07.2019 10:30







English, 03.07.2019 10:30

Mathematics, 03.07.2019 10:30

Social Studies, 03.07.2019 10:30

English, 03.07.2019 10:30





