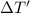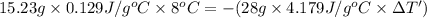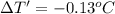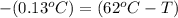Chemistry, 03.11.2019 17:31 lekaje2375
Apiece of gold with a mass of 15.23 g and an initial temperature of 54 °c was dropped into a calorimeter containing 28 g of water. the final temperature of the metal and water in the calorimeter was 62°c. what was the initial temperature of the water?
31.03 °c
62.13 °c
81.03°c
92.13°c

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Apiece of gold with a mass of 15.23 g and an initial temperature of 54 °c was dropped into a calorim...
Questions

English, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00



Mathematics, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00



English, 15.07.2019 16:00

History, 15.07.2019 16:00

History, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00

Geography, 15.07.2019 16:00

Mathematics, 15.07.2019 16:00

English, 15.07.2019 16:00


Mathematics, 15.07.2019 16:00


History, 15.07.2019 16:00

 =62°C-54°C=8°C
=62°C-54°C=8°C