
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfator is added because
O the addition of sulfate ions shifts equilibrium to the left.
O the addition of sodium ions shifts equilibrium to the left.
O the addition of sulfate ions shifts equilibrium to the right. Chd
O the addition of sodium ions shifts equilibrium to the right.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
Strontium sulfate becomes less soluble in an aqueous solution when sodium sulfator is added because...
Questions

History, 18.09.2019 19:10


Mathematics, 18.09.2019 19:10

Mathematics, 18.09.2019 19:10


History, 18.09.2019 19:10


Biology, 18.09.2019 19:10






History, 18.09.2019 19:10

Chemistry, 18.09.2019 19:10

English, 18.09.2019 19:10


Mathematics, 18.09.2019 19:10


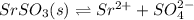
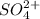 is actually added in to the solution, which causes the equilibrium to shift leftwards according to the Le Ch athelier's principle. Thus, the answer in this case would be:
is actually added in to the solution, which causes the equilibrium to shift leftwards according to the Le Ch athelier's principle. Thus, the answer in this case would be:



