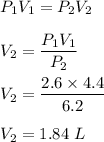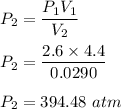Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

You know the right answer?
A sample of air occupies 4.40 L when the pressure is 2.60 atm.
(a) What volume does the sample oc...
Questions


Mathematics, 26.11.2019 01:31


Mathematics, 26.11.2019 01:31

Social Studies, 26.11.2019 01:31

English, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31


Mathematics, 26.11.2019 01:31




History, 26.11.2019 01:31




Mathematics, 26.11.2019 01:31


Social Studies, 26.11.2019 01:31

Mathematics, 26.11.2019 01:31