2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
...

Chemistry, 10.03.2021 04:00 vrentadrienneoqug1a
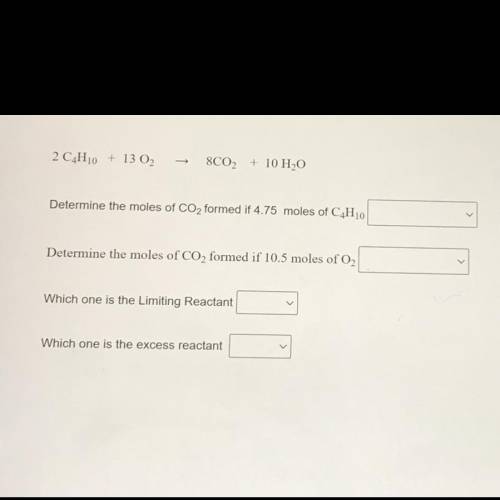
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
<
Determine the moles of CO2 formed if 10.5 moles of O2
Which one is the Limiting Reactant
Which one is the excess reactant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Questions



Social Studies, 03.01.2021 03:00


Mathematics, 03.01.2021 03:00

Chemistry, 03.01.2021 03:00





English, 03.01.2021 03:00

Mathematics, 03.01.2021 03:00

Mathematics, 03.01.2021 03:00


History, 03.01.2021 03:10

Social Studies, 03.01.2021 03:10


Mathematics, 03.01.2021 03:10


Advanced Placement (AP), 03.01.2021 03:10



