
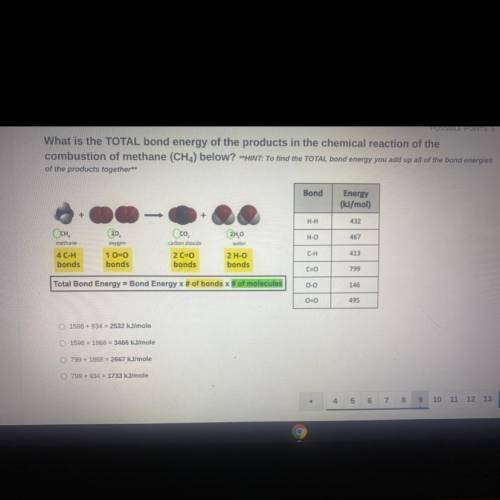
What is the TOTAL bond energy of the products in the chemical reaction of the
combustion of methane (CH4) below? **HINT: To find the TOTAL bond energy you add up all of the bond energies
of the products together**
Bond
Energy
(kJ/mol)
H-H
432
Осн.
H-O
467
methane
Oco
carbon dioxide
2 C=0
bonds
2H,0
water
2 H-O
bonds
oxygen
1 0=0
bonds
413
C-H
4 C-H
bonds
C=0
799
Total Bond Energy = Bond Energy x # of bonds of molecules
0-0
146
00
495
1598 +934 = 2532 kJ/mole
1598 +1868 = 3466 kJ/mole
799 + 1868 = 2667
kJ/mole
799 + 934 = 1733 kJ/mole

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
What is the TOTAL bond energy of the products in the chemical reaction of the
combustion of methane...
Questions

Mathematics, 11.05.2021 02:10

Physics, 11.05.2021 02:10


English, 11.05.2021 02:10


Biology, 11.05.2021 02:10


Mathematics, 11.05.2021 02:10




Mathematics, 11.05.2021 02:10


Mathematics, 11.05.2021 02:10

Mathematics, 11.05.2021 02:10

Mathematics, 11.05.2021 02:10

Social Studies, 11.05.2021 02:10

Mathematics, 11.05.2021 02:10




