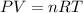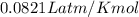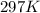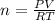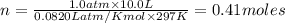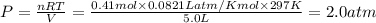Chemistry, 09.03.2021 20:10 2020davidhines
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ideal gas behavior,
what will the pressure be if the same amount of nitrogen
gas is put into a 5.0L container at 297 K?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1



You know the right answer?
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ide...
Questions




Social Studies, 29.01.2021 21:00


Mathematics, 29.01.2021 21:00

English, 29.01.2021 21:00


Chemistry, 29.01.2021 21:00


Mathematics, 29.01.2021 21:00






History, 29.01.2021 21:00


Social Studies, 29.01.2021 21:00