
Chemistry, 09.03.2021 19:20 bellaisbored202
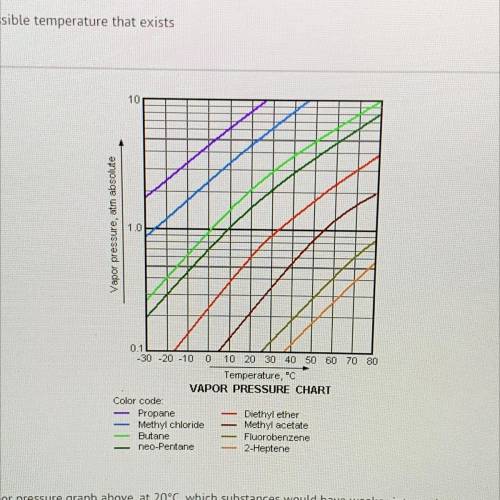
According to the vapor pressure graph above, at 20°C, which substances would have weaker intermolecular forces than diethyl
ether?
A)
butane and propane
B)
butane and 2-heptene
a)
methyl acetate and fluorobenzene
D)
neo pentane and 2-heptene

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1


Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1

Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
According to the vapor pressure graph above, at 20°C, which substances would have weaker intermolecu...
Questions

















Social Studies, 30.06.2019 23:00





