
Chemistry, 09.03.2021 08:50 ashleyroberson735
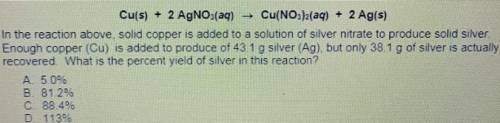
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a solution of silver nitrate to produce solid silver.
Enough copper (Cu) is added to produce of 431 g silver (Ag) but only 38.1 g of silver is actually
recovered What is the percent yield of silver in this reaction?
A. 5.0%
B. 81.2%
C 88 46
D. 1139

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


You know the right answer?
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a sol...
Questions




Computers and Technology, 13.08.2020 22:01




Mathematics, 13.08.2020 22:01


English, 13.08.2020 22:01


Spanish, 13.08.2020 22:01










