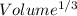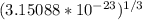Tungsten and molybdenum both have the BCC crystal structure, and Mo forms a substitutional solid solution for all concentrations at room temperature. Compute the unit cell edge length for a 84 wt% W - 16 wt% Mo alloy. The room-temperature density and atomic weight of W are 19.3 g/cm3 and 183.85 g/mol, the room-temperature density and atomic weight of Mo are 10.22 g/cm3 and 95.94 g/mol, respectively.

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
Tungsten and molybdenum both have the BCC crystal structure, and Mo forms a substitutional solid sol...
Questions



Mathematics, 06.11.2019 01:31



Health, 06.11.2019 01:31











Mathematics, 06.11.2019 01:31


Mathematics, 06.11.2019 01:31

Physics, 06.11.2019 01:31