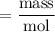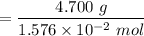Chemistry, 09.03.2021 04:30 lexibyrd120
gA scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr3XBr3 where XX is the unknown metal. The scientist determined that a 4.700 g4.700 g sample of this compound contains 4.834×10−2 mol Br4.834×10−2 mol Br . Calculate the atomic mass of the unknown metal, XX .

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1


Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
gA scientist is trying to discover information about an unknown metal in a compound. The formula for...
Questions

Mathematics, 17.07.2019 08:30

Mathematics, 17.07.2019 08:30

Mathematics, 17.07.2019 08:30


History, 17.07.2019 08:30



Chemistry, 17.07.2019 08:30


Mathematics, 17.07.2019 08:30


English, 17.07.2019 08:30







Mathematics, 17.07.2019 08:30

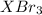
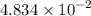 mol of Br.
mol of Br. 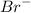 contain in 1 mol of
contain in 1 mol of 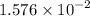 mol of
mol of 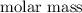 of
of