
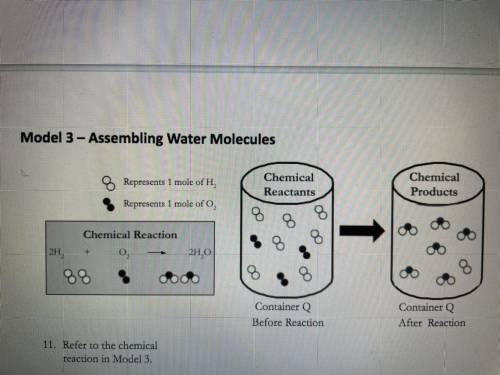
Consider the synthesis of water as shown in Model 3. A container is filled with 10.0 g of H, and
5.0 g of O,
a. Which reactant (hydrogen or oxygen) is the limiting reactant in this case? Show your work.
Hint: Notice that you are given reactant quantities in mass units here, not moles.
b. What mass of water can be produced? Show your work.
c. Which reactant is present in excess, and what mass of that reactant remains after the
reaction is complete? Show your work.
I

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3


Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Consider the synthesis of water as shown in Model 3. A container is filled with 10.0 g of H, and
5....
Questions



Mathematics, 24.09.2019 09:00


Social Studies, 24.09.2019 09:00


Mathematics, 24.09.2019 09:00



Mathematics, 24.09.2019 09:00


Physics, 24.09.2019 09:00

Mathematics, 24.09.2019 09:00


History, 24.09.2019 09:00







