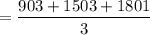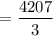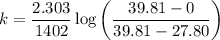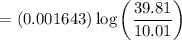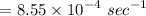3. Methyl acetate is hydrolyzed at 25 oC in acidic environment. Aliquots of equal volume are removed and titrated with NaOH solution. The hydrolysis reaction is irreversible. The results are Time/s 339 1242 2745 4546 infinite Volume of base/ mL 26.34 27.80 29.70 31.81 39.81 Write the reaction of hydrolysis of methyl acetate. (3 pts.) Neglect the reverse reaction. Find the order of the hydrolysis reaction and the value of the rate constant at this temperature. (12 pts.) Total 15 pts.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
3. Methyl acetate is hydrolyzed at 25 oC in acidic environment. Aliquots of equal volume are removed...
Questions

Mathematics, 07.07.2019 13:00

Computers and Technology, 07.07.2019 13:00





Mathematics, 07.07.2019 13:00



Chemistry, 07.07.2019 13:00

Chemistry, 07.07.2019 13:00

English, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

History, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

History, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00


History, 07.07.2019 13:00

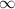 39.81
39.81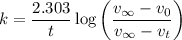
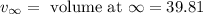
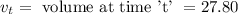
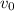 = volume at time 0 = 0
= volume at time 0 = 0