
Chemistry, 09.03.2021 03:10 shaylaahayden45061
PLEASE HELP I'VE BEEN STUCK ON THIS FOR 2 HOURS
Calculate the mass of water produced from the reaction between 8.50 g of Hydrogen gas and 85.0 grams of Oxygen gas.
(You're going to need a periodic table, and this is Mass to Mass conversion)
Equation: 2H2O->2H2 + O2
I got the answer 180.7 gH202 but it's not correct.
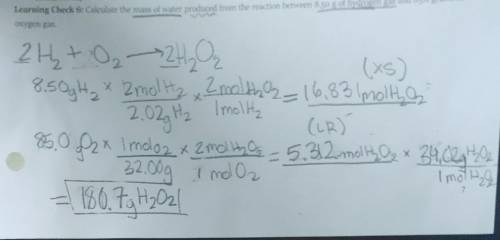

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
PLEASE HELP I'VE BEEN STUCK ON THIS FOR 2 HOURS
Calculate the mass of water produced from the react...
Questions


Biology, 11.06.2021 20:40

Geography, 11.06.2021 20:40

Mathematics, 11.06.2021 20:40

Mathematics, 11.06.2021 20:40



Mathematics, 11.06.2021 20:40

Mathematics, 11.06.2021 20:40


Mathematics, 11.06.2021 20:40


Biology, 11.06.2021 20:40


English, 11.06.2021 20:40

Mathematics, 11.06.2021 20:40

English, 11.06.2021 20:40


Mathematics, 11.06.2021 20:40

Mathematics, 11.06.2021 20:40



