
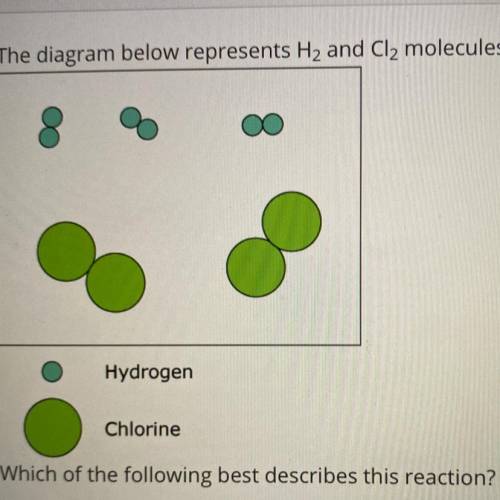
The diagram below represents H2 and Cl2 molecules that will potentially react to form HCI.
Hydrogen
Chlorine
Which of the following best describes this reaction?
А
The limiting reactant is chlorine.
B
The percent yield will be greater than 100%.
с
Hydrogen and chlorine are nonreactive.
D
All molecules shown will react to form the product.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
The diagram below represents H2 and Cl2 molecules that will potentially react to form HCI.
Hydrogen...
Questions



Mathematics, 29.06.2019 10:20


Mathematics, 29.06.2019 10:20

Computers and Technology, 29.06.2019 10:20

Mathematics, 29.06.2019 10:20

Health, 29.06.2019 10:20





Mathematics, 29.06.2019 10:20



History, 29.06.2019 10:20


History, 29.06.2019 10:20


Business, 29.06.2019 10:20



