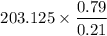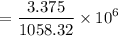Chemistry, 08.03.2021 20:10 nikitakhrabkov123
A mixture of 75 mole% methane and 25 mole% hydrogen is burned with 25% excess air. Fractional conversions of 90% of the methane and 85% of the hydrogen are achieved; of the methane that reacts, 95% reacts to form CO2 and the balance reacts to form CO. The hot combustion product gas passes through a boiler in which heat transferred from the gas converts boiler feedwater into steam.
Required:
a. Calculate the concentration of CO (ppm) in the stack gas.
b. The CO in the stack gas is a pollutant. Its concentration can be decreased by increasing the percent excess air fed to the furnace. Think of at least two costs of doing so. (Hint: The heat released by the combustion goes into heating the combustion products; the higher the combustion product temperature, the more steam is produced.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

You know the right answer?
A mixture of 75 mole% methane and 25 mole% hydrogen is burned with 25% excess air. Fractional conver...
Questions



Mathematics, 07.05.2021 19:40




Mathematics, 07.05.2021 19:40

Computers and Technology, 07.05.2021 19:40

Spanish, 07.05.2021 19:40

Computers and Technology, 07.05.2021 19:40


Chemistry, 07.05.2021 19:40



Mathematics, 07.05.2021 19:40

Mathematics, 07.05.2021 19:40


Mathematics, 07.05.2021 19:40


Computers and Technology, 07.05.2021 19:40

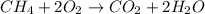
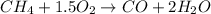
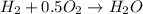
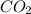 formed is = 0.95 x 67.5
formed is = 0.95 x 67.5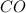 formed is = 0.05 x 67.5
formed is = 0.05 x 67.5