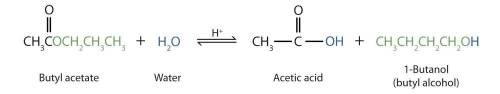
Esters may undergo a process called hydrolysis in which an ester is split by water. Hydrolysis of esters can occur in the presence of strong acids or strong bases. When heated in the presence of a strong acid such as H2SO4, water splits an ester into a carboxylic acid and an alcohol. This process is called acid hydrolysis. In the presence of a strong base such as NaOH, water splits an ester into a carboxylic acid salt and an alcohol. This process is called base hydrolysis or saponification.
Required:
Draw the carboxylic acid produced from the acid hydrolysis of butyl acetate.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Esters may undergo a process called hydrolysis in which an ester is split by water. Hydrolysis of es...
Questions




History, 21.11.2019 16:31


World Languages, 21.11.2019 16:31



Biology, 21.11.2019 16:31

Mathematics, 21.11.2019 16:31



Geography, 21.11.2019 16:31

History, 21.11.2019 16:31

Mathematics, 21.11.2019 16:31

Mathematics, 21.11.2019 16:31

Mathematics, 21.11.2019 16:31



Mathematics, 21.11.2019 16:31