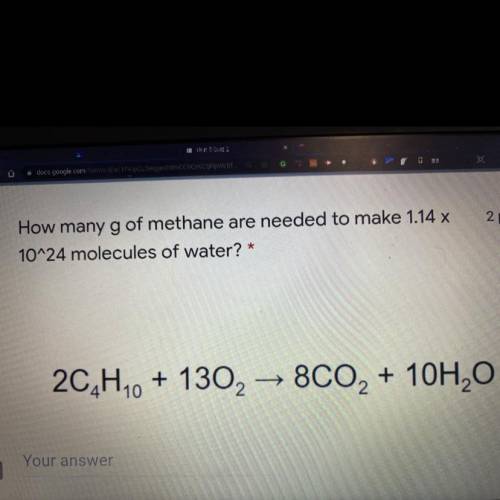How many g of methane are needed to make 1.14 x 10^24 molecules of
water? *
2C4H10 + 13O2 → 8...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Questions







Mathematics, 11.06.2020 19:57

Mathematics, 11.06.2020 19:57

Biology, 11.06.2020 19:57

Mathematics, 11.06.2020 19:57








Mathematics, 11.06.2020 19:57

Social Studies, 11.06.2020 19:57