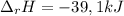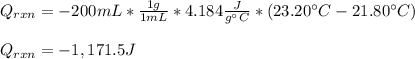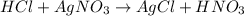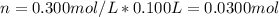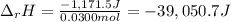100 mL of a 0.300 M solution of AgNO3 reacts with 100 mL of a 0.300 M solution of HCl in a
coffee-cup calorimeter and the temperature rises from 21.80 °C to 23.20 °C. Assuming the density
and specific heat of the resulting solution is 1.00 g/mL and 4.18 J/g. °C, respectfully, what is the
AHºx?
A 39.0 kJ/mol
B +39.0 kJ/mol
C.+1.17 kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3


Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
100 mL of a 0.300 M solution of AgNO3 reacts with 100 mL of a 0.300 M solution of HCl in a
coffee-c...
Questions


History, 26.01.2021 21:10

Mathematics, 26.01.2021 21:10


Mathematics, 26.01.2021 21:10





Mathematics, 26.01.2021 21:10


History, 26.01.2021 21:10

Mathematics, 26.01.2021 21:10

Biology, 26.01.2021 21:10


Geography, 26.01.2021 21:10


Social Studies, 26.01.2021 21:10