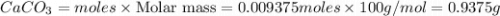Show
your calculations for full marks
1. Calculate the mass of CaCO3(s) required to react wit...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
Questions


Mathematics, 24.10.2019 10:43


Biology, 24.10.2019 10:43







Mathematics, 24.10.2019 10:43

History, 24.10.2019 10:43

World Languages, 24.10.2019 10:43


History, 24.10.2019 10:43





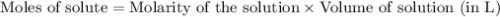 .....(1)
.....(1)
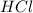 solution = 0.75 M
solution = 0.75 M
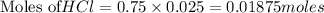
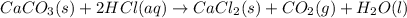

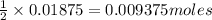 of
of